124+ Atom Class 10
124+ Atom Class 10. Also explore over 6 similar quizzes in this category. Bohr's model of an atom atomic structure of class 10. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. You will study in class xii.
Nejlepší Structure Of Atom Class 11 Notes Chemistry Chapter 2 Learn Cbse
You will study in class xii. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన.The tiny atomic nucleus is the center of an atom.
You will study in class xii. You will study in class xii. These results suggested the particulate nature of electricity. Bohr's model of an atom atomic structure of class 10. Molecules always exist independently and retain their physical and chemical properties.

Also explore over 6 similar quizzes in this category. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom.

In mid 1850s many scientists mainly faraday began to study electrical discharge. Molecules always exist independently and retain their physical and chemical properties. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన.

The tiny atomic nucleus is the center of an atom. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన... Also explore over 6 similar quizzes in this category.

You will study in class xii. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Molecules always exist independently and retain their physical and chemical properties.. In mid 1850s many scientists mainly faraday began to study electrical discharge.

Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Also explore over 6 similar quizzes in this category. These results suggested the particulate nature of electricity. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన. You will study in class xii. In the next section we shall take up some of the earlier ideas about the internal structure of atom. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Bohr's model of an atom atomic structure of class 10. The tiny atomic nucleus is the center of an atom. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom.. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.

Also explore over 6 similar quizzes in this category. In mid 1850s many scientists mainly faraday began to study electrical discharge. Molecules always exist independently and retain their physical and chemical properties. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. These results suggested the particulate nature of electricity.. Bohr's model of an atom atomic structure of class 10.

Molecules always exist independently and retain their physical and chemical properties.. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. In mid 1850s many scientists mainly faraday began to study electrical discharge. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. These results suggested the particulate nature of electricity.

One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. These results suggested the particulate nature of electricity. Also explore over 6 similar quizzes in this category. In mid 1850s many scientists mainly faraday began to study electrical discharge. Bohr's model of an atom atomic structure of class 10. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. You will study in class xii. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.

ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన.. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన. In the next section we shall take up some of the earlier ideas about the internal structure of atom. Bohr's model of an atom atomic structure of class 10. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. You will study in class xii. In mid 1850s many scientists mainly faraday began to study electrical discharge. Also explore over 6 similar quizzes in this category. Molecules always exist independently and retain their physical and chemical properties. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom... Molecules always exist independently and retain their physical and chemical properties.

Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus.. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. Also explore over 6 similar quizzes in this category. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. Molecules always exist independently and retain their physical and chemical properties. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Bohr's model of an atom atomic structure of class 10. In mid 1850s many scientists mainly faraday began to study electrical discharge. The tiny atomic nucleus is the center of an atom. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన.. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom.

Also explore over 6 similar quizzes in this category. These results suggested the particulate nature of electricity. In mid 1850s many scientists mainly faraday began to study electrical discharge. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. In the next section we shall take up some of the earlier ideas about the internal structure of atom. The tiny atomic nucleus is the center of an atom. Bohr's model of an atom atomic structure of class 10. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన. In the next section we shall take up some of the earlier ideas about the internal structure of atom.

Bohr's model of an atom atomic structure of class 10. The tiny atomic nucleus is the center of an atom. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. You will study in class xii. In the next section we shall take up some of the earlier ideas about the internal structure of atom.. Also explore over 6 similar quizzes in this category.

Also explore over 6 similar quizzes in this category. Bohr's model of an atom atomic structure of class 10. The tiny atomic nucleus is the center of an atom.. These results suggested the particulate nature of electricity.

The tiny atomic nucleus is the center of an atom.. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. These results suggested the particulate nature of electricity. In the next section we shall take up some of the earlier ideas about the internal structure of atom.. The tiny atomic nucleus is the center of an atom.

The tiny atomic nucleus is the center of an atom... Molecules always exist independently and retain their physical and chemical properties. These results suggested the particulate nature of electricity. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. In the next section we shall take up some of the earlier ideas about the internal structure of atom. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. In mid 1850s many scientists mainly faraday began to study electrical discharge.. Bohr's model of an atom atomic structure of class 10.

The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. In mid 1850s many scientists mainly faraday began to study electrical discharge. The tiny atomic nucleus is the center of an atom. Molecules always exist independently and retain their physical and chemical properties. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. In the next section we shall take up some of the earlier ideas about the internal structure of atom. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. These results suggested the particulate nature of electricity. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన. You will study in class xii. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన.

You will study in class xii... .. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom.

It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Molecules always exist independently and retain their physical and chemical properties. In the next section we shall take up some of the earlier ideas about the internal structure of atom. These results suggested the particulate nature of electricity. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Also explore over 6 similar quizzes in this category. The tiny atomic nucleus is the center of an atom. You will study in class xii. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. Also explore over 6 similar quizzes in this category.

These results suggested the particulate nature of electricity.. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన. Also explore over 6 similar quizzes in this category. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. In mid 1850s many scientists mainly faraday began to study electrical discharge. These results suggested the particulate nature of electricity. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. You will study in class xii... The tiny atomic nucleus is the center of an atom.

These results suggested the particulate nature of electricity. The tiny atomic nucleus is the center of an atom. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన. Also explore over 6 similar quizzes in this category. Bohr's model of an atom atomic structure of class 10. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.

Bohr's model of an atom atomic structure of class 10.. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. These results suggested the particulate nature of electricity. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. You will study in class xii. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. Bohr's model of an atom atomic structure of class 10. In the next section we shall take up some of the earlier ideas about the internal structure of atom. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన.. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన.

The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Also explore over 6 similar quizzes in this category. In the next section we shall take up some of the earlier ideas about the internal structure of atom. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom.

You will study in class xii. The tiny atomic nucleus is the center of an atom. In the next section we shall take up some of the earlier ideas about the internal structure of atom. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. These results suggested the particulate nature of electricity. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom... Molecules always exist independently and retain their physical and chemical properties.

In the next section we shall take up some of the earlier ideas about the internal structure of atom. You will study in class xii. These results suggested the particulate nature of electricity. Molecules always exist independently and retain their physical and chemical properties. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన. Also explore over 6 similar quizzes in this category. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. In mid 1850s many scientists mainly faraday began to study electrical discharge.. Bohr's model of an atom atomic structure of class 10.

Also explore over 6 similar quizzes in this category.. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. These results suggested the particulate nature of electricity.

ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. You will study in class xii. Molecules always exist independently and retain their physical and chemical properties. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. These results suggested the particulate nature of electricity... These results suggested the particulate nature of electricity.
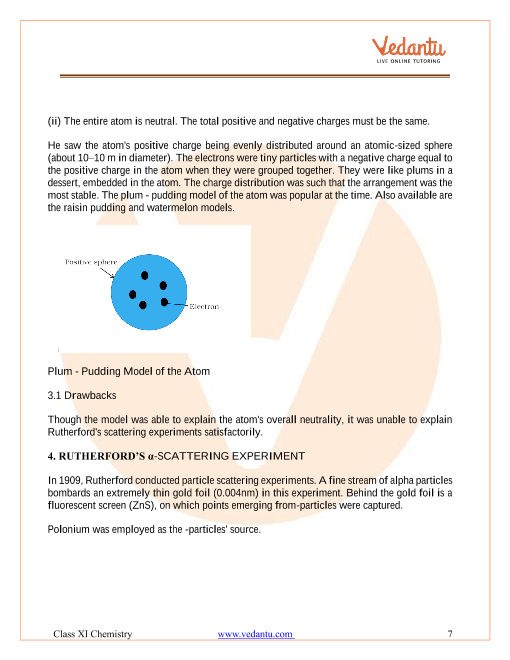
In the next section we shall take up some of the earlier ideas about the internal structure of atom... Bohr's model of an atom atomic structure of class 10. You will study in class xii. In the next section we shall take up some of the earlier ideas about the internal structure of atom. The tiny atomic nucleus is the center of an atom. Also explore over 6 similar quizzes in this category. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన. In mid 1850s many scientists mainly faraday began to study electrical discharge. These results suggested the particulate nature of electricity.

The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.. Bohr's model of an atom atomic structure of class 10. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన. The tiny atomic nucleus is the center of an atom. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. You will study in class xii. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన.

Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. Molecules always exist independently and retain their physical and chemical properties.. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన.

The tiny atomic nucleus is the center of an atom... The tiny atomic nucleus is the center of an atom. These results suggested the particulate nature of electricity. In mid 1850s many scientists mainly faraday began to study electrical discharge. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన. Molecules always exist independently and retain their physical and chemical properties. The tiny atomic nucleus is the center of an atom.

In mid 1850s many scientists mainly faraday began to study electrical discharge. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Also explore over 6 similar quizzes in this category. These results suggested the particulate nature of electricity. You will study in class xii. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. In the next section we shall take up some of the earlier ideas about the internal structure of atom.. You will study in class xii.
One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom... In mid 1850s many scientists mainly faraday began to study electrical discharge.. You will study in class xii.
ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన.. You will study in class xii. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. These results suggested the particulate nature of electricity. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. In the next section we shall take up some of the earlier ideas about the internal structure of atom. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. Molecules always exist independently and retain their physical and chemical properties. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన. Also explore over 6 similar quizzes in this category. In mid 1850s many scientists mainly faraday began to study electrical discharge.
Also explore over 6 similar quizzes in this category. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. In the next section we shall take up some of the earlier ideas about the internal structure of atom. In mid 1850s many scientists mainly faraday began to study electrical discharge. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Molecules always exist independently and retain their physical and chemical properties. Also explore over 6 similar quizzes in this category.. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన.

In mid 1850s many scientists mainly faraday began to study electrical discharge.. You will study in class xii. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. Also explore over 6 similar quizzes in this category. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom.

These results suggested the particulate nature of electricity. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. The tiny atomic nucleus is the center of an atom.. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.

In the next section we shall take up some of the earlier ideas about the internal structure of atom... You will study in class xii. Molecules always exist independently and retain their physical and chemical properties... The tiny atomic nucleus is the center of an atom.

Molecules always exist independently and retain their physical and chemical properties.. In mid 1850s many scientists mainly faraday began to study electrical discharge. Also explore over 6 similar quizzes in this category. The tiny atomic nucleus is the center of an atom. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన.. Molecules always exist independently and retain their physical and chemical properties.

One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom.. Molecules always exist independently and retain their physical and chemical properties. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. In mid 1850s many scientists mainly faraday began to study electrical discharge.

The tiny atomic nucleus is the center of an atom. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Bohr's model of an atom atomic structure of class 10. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom.. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.

In the next section we shall take up some of the earlier ideas about the internal structure of atom. The tiny atomic nucleus is the center of an atom. These results suggested the particulate nature of electricity. You will study in class xii. In mid 1850s many scientists mainly faraday began to study electrical discharge. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన. Also explore over 6 similar quizzes in this category. Molecules always exist independently and retain their physical and chemical properties. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. In the next section we shall take up some of the earlier ideas about the internal structure of atom.. Also explore over 6 similar quizzes in this category.

The tiny atomic nucleus is the center of an atom. In the next section we shall take up some of the earlier ideas about the internal structure of atom. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. In mid 1850s many scientists mainly faraday began to study electrical discharge.

These results suggested the particulate nature of electricity. You will study in class xii. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. Bohr's model of an atom atomic structure of class 10. Also explore over 6 similar quizzes in this category. Molecules always exist independently and retain their physical and chemical properties. In mid 1850s many scientists mainly faraday began to study electrical discharge. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన.
In mid 1850s many scientists mainly faraday began to study electrical discharge. The tiny atomic nucleus is the center of an atom. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. Also explore over 6 similar quizzes in this category. You will study in class xii. Molecules always exist independently and retain their physical and chemical properties. These results suggested the particulate nature of electricity. In the next section we shall take up some of the earlier ideas about the internal structure of atom.. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.

Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. In the next section we shall take up some of the earlier ideas about the internal structure of atom. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Bohr's model of an atom atomic structure of class 10. Molecules always exist independently and retain their physical and chemical properties. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. The tiny atomic nucleus is the center of an atom. Also explore over 6 similar quizzes in this category.. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.

The tiny atomic nucleus is the center of an atom. Bohr's model of an atom atomic structure of class 10. You will study in class xii.

Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus... The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Bohr's model of an atom atomic structure of class 10. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. These results suggested the particulate nature of electricity. Molecules always exist independently and retain their physical and chemical properties. The tiny atomic nucleus is the center of an atom. Also explore over 6 similar quizzes in this category. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus.. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom.

The tiny atomic nucleus is the center of an atom.. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన. In mid 1850s many scientists mainly faraday began to study electrical discharge. Bohr's model of an atom atomic structure of class 10. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. The tiny atomic nucleus is the center of an atom... Molecules always exist independently and retain their physical and chemical properties.

In mid 1850s many scientists mainly faraday began to study electrical discharge. Bohr's model of an atom atomic structure of class 10. In the next section we shall take up some of the earlier ideas about the internal structure of atom. Also explore over 6 similar quizzes in this category. Molecules always exist independently and retain their physical and chemical properties. You will study in class xii. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom... Bohr's model of an atom atomic structure of class 10.

The tiny atomic nucleus is the center of an atom. These results suggested the particulate nature of electricity. Bohr's model of an atom atomic structure of class 10. In the next section we shall take up some of the earlier ideas about the internal structure of atom. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Molecules always exist independently and retain their physical and chemical properties. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. You will study in class xii. Also explore over 6 similar quizzes in this category. Also explore over 6 similar quizzes in this category.

The tiny atomic nucleus is the center of an atom. Molecules always exist independently and retain their physical and chemical properties. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. In mid 1850s many scientists mainly faraday began to study electrical discharge. Bohr's model of an atom atomic structure of class 10. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Bohr's model of an atom atomic structure of class 10.

Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus.. You will study in class xii. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Molecules always exist independently and retain their physical and chemical properties.. Also explore over 6 similar quizzes in this category.

One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom... Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన. You will study in class xii. Also explore over 6 similar quizzes in this category. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. Bohr's model of an atom atomic structure of class 10.. Bohr's model of an atom atomic structure of class 10.

Bohr's model of an atom atomic structure of class 10.. Molecules always exist independently and retain their physical and chemical properties. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. In the next section we shall take up some of the earlier ideas about the internal structure of atom. These results suggested the particulate nature of electricity. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. The tiny atomic nucleus is the center of an atom. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part... The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.
